Program Details
Enriching local talent through the development of fundamental biostatistics and pharmacometrics skills.
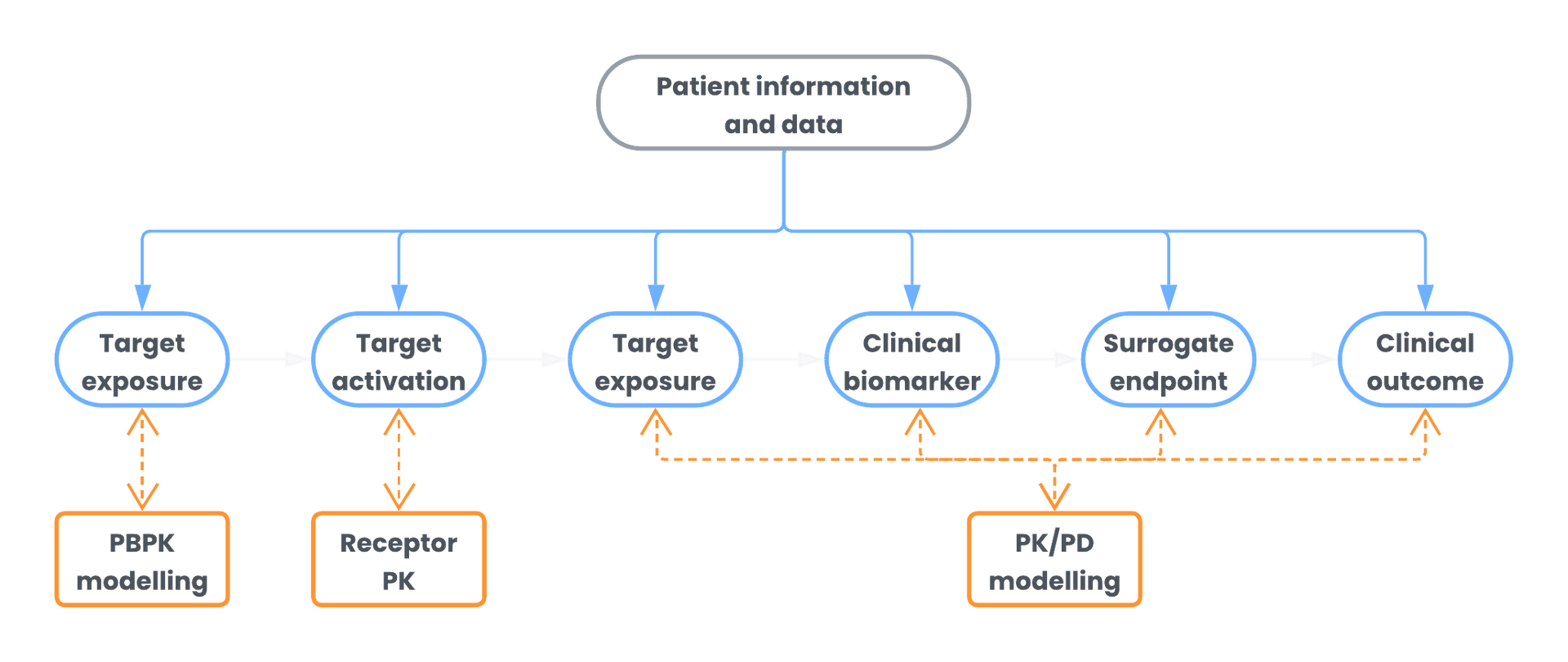
API’s Pharmacometrics fellowship was designed to foster talent development by supporting the growth of a skilled pharmacometrics workforce. This program emphasizes statistical and pharmacokinetic-pharmacodynamic (PKPD) models, quantitative systems toxicology and pharmacology, and advanced drug modeling.
By combining rigorous training, cutting-edge curriculum, and real-world experience, the API Pharmacometrics Fellowship propels research advancement through patient-focused individualization of drug therapy. This allows for improved clinical outcomes that contribute to drug development via clinical study enrichment.

Goals & Objectives
What will you gain?
-
Skills to develop and assess PKPD models
-
An understanding of regulatory requirements for clinical pharmacokinetics
-
Aptitude in statistical and study design
-
Practice in model-informed drug development through modeling and simulation
-
Understanding the integration of quantitative systems pharmacology and machine learning
-
Application of acquired skills in a real-world drug development project
Areas of Study
Delivered in person and via API’s learning management system, Absorb, the Pharmacometrics Fellowship is a two-part series of lectures, presented by API staff and partners at the University of Alberta.
Part 1
Pharmacokinetics
Presented by Micheal Guirguis and Raimar Loebenberg.
In part one, students will learn from lectures focused in each of the following subject areas:
- General Introduction to Pharmacokinetics
- IV bolus injection
- Metabolism, Excretion, Distribution – Part 1
- Routes of Administration
- Pharmacokinetics following Oral Administration
- Concepts surrounding BCS Classification
- The Role of the Formulation
- Bioequivalence Study
- Metabolism, Excretion and Distribution – Part 2
- Clinical Pharmacology – Phase 1 Studies Summary
Part 2
Biostatistics
Presented by Morteza Hajihosseini, Fernanda Talarico, Nastaran Hajizadeh, and Erfan Ghasemi.
In part two, students will learn from lectures focused in each of the following subject areas:
- Introduction to Biostatistics, Exploratory Data Analysis, Probability and Sampling Distribution
- Estimation, Hypothesis Testing, and Maximum Likelihood Estimator (MLE)
- Score Function, Hessian, Fisher Information, Quadratic Approximation and Standard Error
- Wald Confidence Intervals, Likelihood Ratio Tests, and Model Selection
- Akaike and Bayesian Information Criteria (AIC & BIC), and maximising the likelihood by the Newton’s Method
- Mixed Effects Models
- Approximating the integrals, Laplace and First Order (FO & FOCE and FOCE-Interaction) Approximations, Numerical Quadrature
- The Expectation Maximisation (EM) Algorithm
- MU-Modelling, Iterative Two Stage (ITS)
- Monte Carlo EM (MCEM), Importance Sampling, Direct Sampling, SAEM, Markov Chain Monte Carlo (MCMC)
- Estimating the random effects, empirical bayes estimates (EBE) and shrinkage
- Asymptotic properties of the MLE, efficiency, consistency, normality, and the Cramer-Rao Lower Bound (CRLB)
- Robustness of the MLE and the Kullback-Liebler distance
Part 3
Modeling and Simulation
Presented by Patrick Mayo and Caroline Zhao.
In part three, students will learn from lectures focused in each of the following subject areas:
- Compartmental PK Part 1 – Introduction Basic concepts
- Compartmental PK – Part 2 – Approaches to Modeling/Statistical Methods
- Compartmental PK – Part 3 – Introduction to Population PK Modeling
- Compartmental PK – Part 4 Population PK Modeling – Software and Technical issues
- Introduction to PK/PD
Stay updated
This program is suited for graduate students in Pharmaceutical Sciences, Biological Sciences, Biostatistics, Applied Mathematics, Epidemiology, or related fields, as well as PharmD or practicing healthcare professionals with an interest in pharmacometrics and drug development. For inquiries or more information about the program, contact pk.fellowship@appliedpharma.ca.
If you’re interested and eligible for future cycles of the program, sign up to our monthly newsletter below and be the first to know about application and registration dates.

